Research
Pluripotency Exit
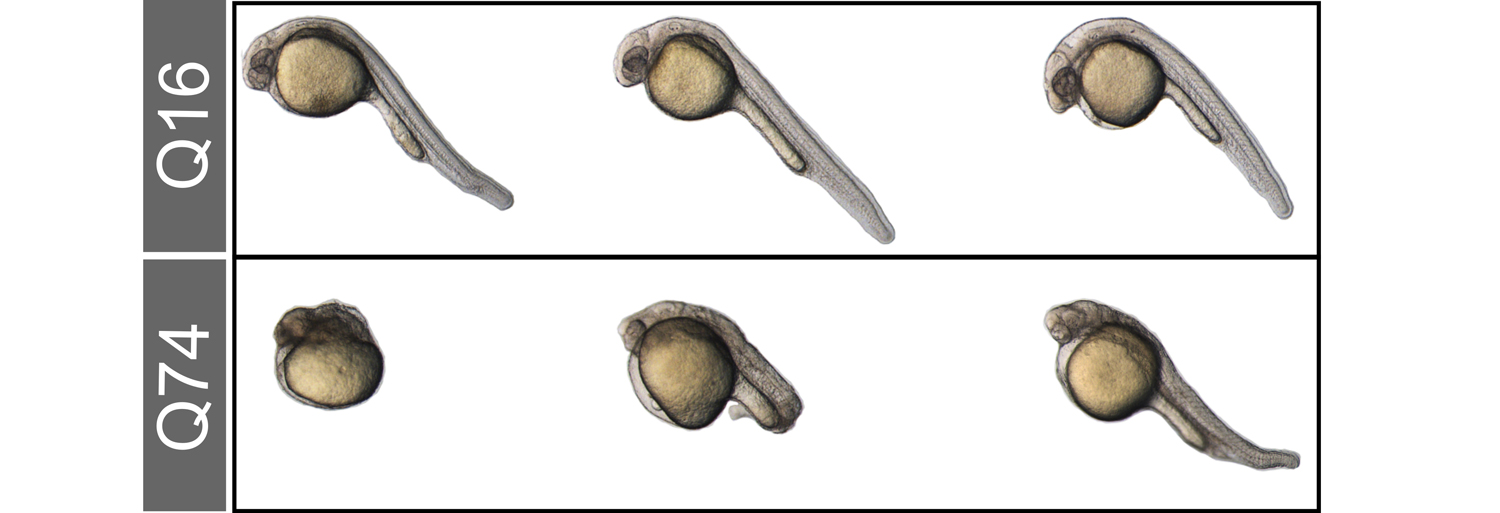
In the mouse embryo, naïve pluripotent cells of the epiblast form before embryo implantation. During the following 24 hours pluripotent epiblast cells undergo morphological and transcriptional changes in preparation for their irreversible exit from the pluripotent state and their differentiation into more specialized cells of the germline and the three germ layers. We are studying factors guiding pluripotent cells through such a series of molecular changes.
To know more: Smith A. 2017 doi ; Carbognin et al., 2020 .
Intracellular Communication
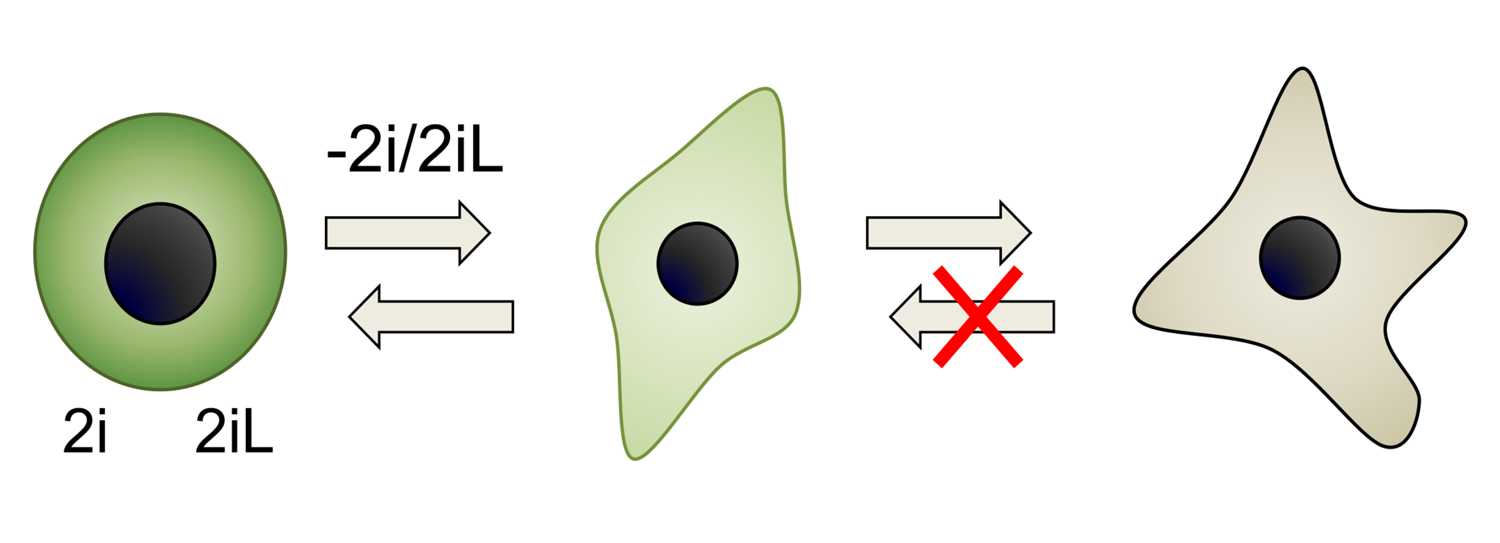
The cytokine LIF and its downstream transcription factor Stat3 play crucial roles in induction and maintenance of pluripotency. Stat3 has been reported to localise to different organelles, exerting different effects on the cell. We reported that Stat3 controls both nuclear transcription and mitochondrial activity in pluripotent cells. We are currently studying whether and how the two organelles communicate and we are also interested in the evolutionary conservation of such molecular activities.
To know more: Carbognin et al., 2016 doi ; Peron et al., 2020 doi .
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 716910).
Human Pluripotency Building Blocks
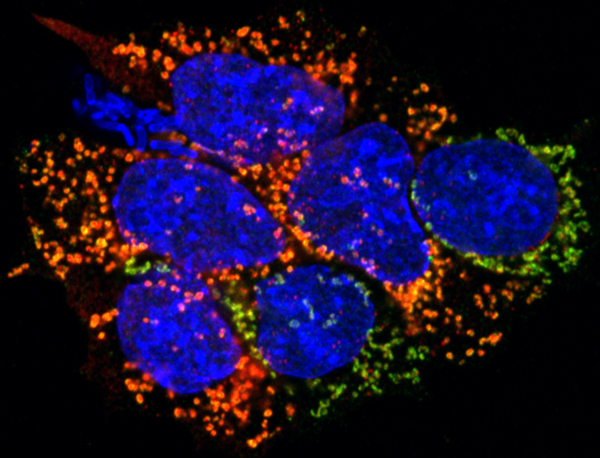
Human PSCs, such as ES and iPS cells are commonly used as in vitro models of early development and also for disease modelling. However, human iPS cells still show abnormalities and differentiation biases which might limit their use. Our aim is to characterise the signals and factors controlling human pluripotency with the aim of overcoming some of the current limitations of human PSCs.
To know more: Zorzan et al., 2020 doi ; Perrera et al., 2020 doi .
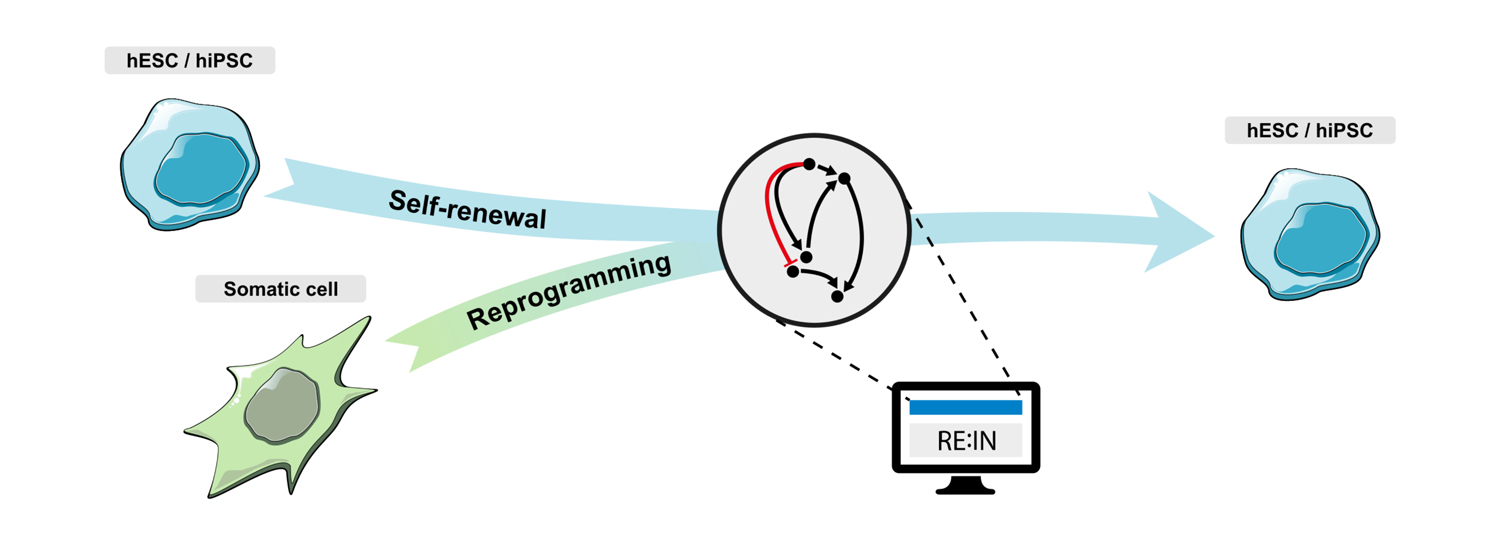
In Silico Stem Cells
In collaboration with MSR we developed a model of murine pluripotency network and exploited it to identify the logic and factors controlling induction of pluripotency in murine cells. We are currently working on a similar approach for the study of human pluripotency, with the aim of optimizing the generation and differentiation of human pluripotent cells.
To know more: Dunn et al., 2014 doi ; Dunn et al., 2019 doi ; Yordanov et al., 2016 doi
Studying Neurodegeneration with Stem Cells
PSCs represent a valuable experimental tool, given the possibility to genetically modify them and their ability to differentiate in vitro into virtually any cell type. For these reasons we are modelling Huntington disease (HD) in PSCs with the aim to identify genes involved in the cellular toxicity caused by mutant Huntingtin, the protein responsible for HD. We combine genome-wide genetic approaches and bioinformatics to identify genes involved in HD development and test them as potential therapeutic targets in in vivo HD models.